Vax2Muc research highlights: from nanoparticles to preclinical models
The Vax2Muc consortium aims to develop next-generation vaccines against diseases caused by mucosal pathogens colonising the gastrointestinal tract. More concretely, the goal of this interdisciplinary research collaboration is to create, as a proof-of-concept, a prophylactic H. pylori vaccine candidate that will be evaluated in a phase I clinical trial. Two years into the project, results are starting to emerge, and the researchers are busy presenting their findings at scientific conferences. This year, three posters have been presented so far, each of them bringing more insight towards the overall goal of new vaccine development.
Vax2Muc is aiming to develop a mucosal vaccine, triggering local immunity in the stomach, where pathogens such as H. pylori enter the body. Researchers are exploring innovative technologies that will ensure a vaccine remains effective in the highly specific environment of the stomach. The posters presented so far this year have focused on the methods for transferring a vaccine to the stomach without degradation, as well as on the future testing of vaccine candidates.
Conference: 5th European Conference on Pharmaceutics, Porto, Portugal
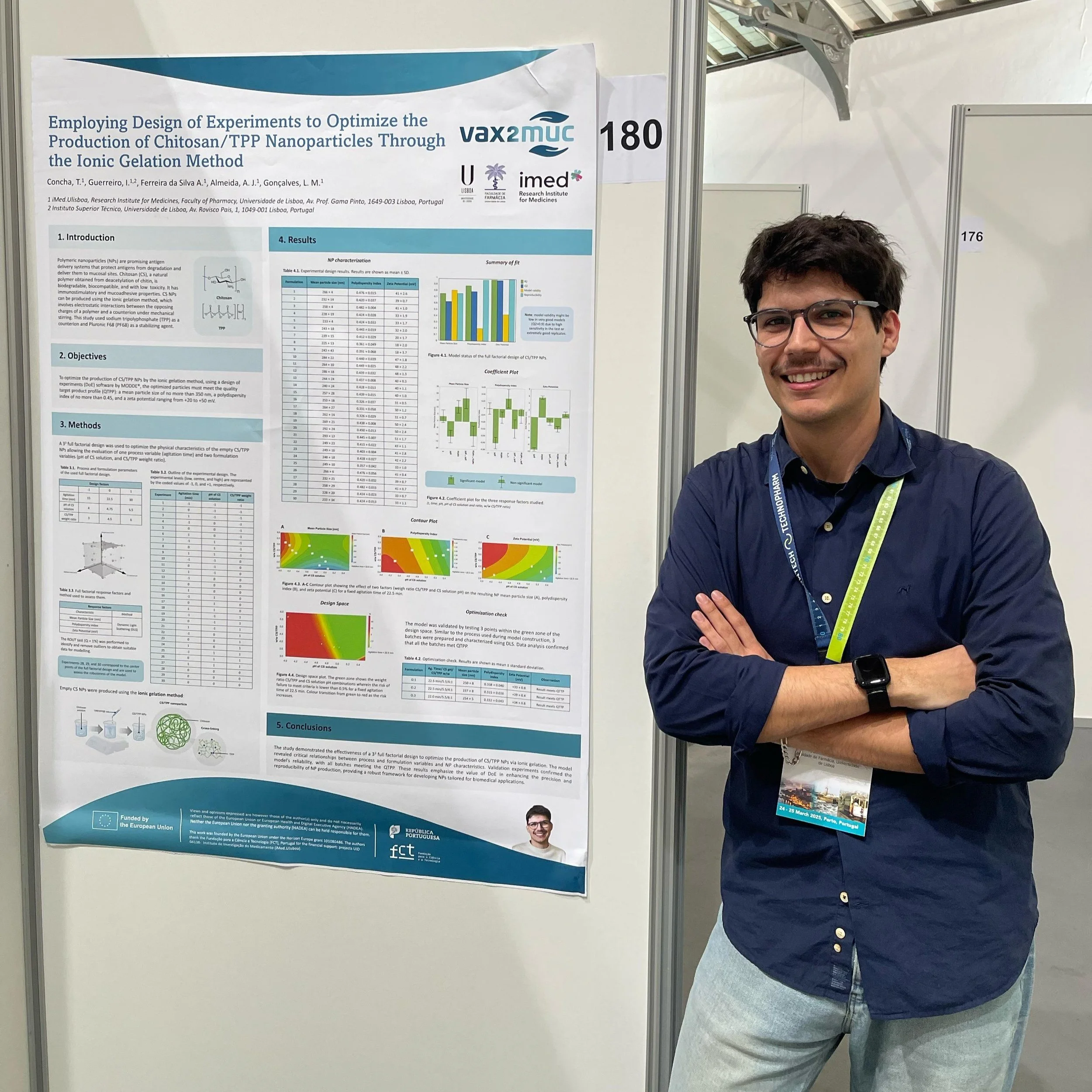
Poster title: Employing Design of Experiments to Optimize the Production of Chitosan/TPP Nanoparticles Through the Ionic Gelation Method
Presenter: Tomás Almeida, PhD student, University of Lisbon’s Faculty of Pharmacy
Tomás Almeida at the European Conference on Pharmaceutics 2025
What is this poster about?
The poster presents findings from a Vax2Muc study that aimed to find the best way to make chitosan nanoparticles using the ionic gelation method.
Chitosan nanoparticles, due to their positive charge, help deliver vaccines or drugs to mucosal sites by adhering to the surface of cells in the gastrointestinal tract.
Researchers aimed to create nanoparticles that meet specific quality standards, known as the Quality Target Product Profile by using a scientific approach called Design of Experiments, specifically a "3^3 full factorial design".
Design of Experiments is a data collection and analysis tool that helps researchers run experiments in a planned and efficient way, making it easier to understand the effects of one or more factors.
This allowed them to systematically test all possible combinations between three factors (pH, mass ratio, and agitation time) at three different levels (high, medium, and low) to see how the factors affected the nanoparticle characteristics and better understand how to create nanoparticles that can be used in ongoing Vax2Muc research.
What are the main findings?
The study demonstrated that using Design of Experiments is a valuable way to precisely and consistently produce chitosan nanoparticles. Researchers were able to identify combinations of the tested factors that would most likely produce nanoparticles that met all the desired quality criteria. They then produced three additional batches of nanoparticles using optimal parameters.
All three of these validation batches met the Quality Target Product Profile.
How do these findings fit into the ongoing Vax2Muc research?
Vax2Muc aims to develop a mucosal vaccine which will trigger local immunity and prevent pathogens from entering the body and causing disease.
Bacteria like H. pylori – the focus of Vax2Muc research – enter the body through the stomach, and so a successful vaccine formulation will have to survive the stomach’s harsh environment.
Research into nanoparticles which can help deliver the vaccine to its destination without degradation is a crucial step in this scientific endeavour.
Interested in finding out more?
Download the poster here.
Conference: European Symposium of Porcine Health Management (ESPHM 2025), Bern, Switzerland
Poster title: Frequency of detection of Helicobacter suis and Helicobacter pylori in healthy pigs of different ages in Spain
Presenter: Freddy Dehesa-Canseco, PhD student, Institute of Agrifood Research and Technology’s Animal Health Research Center (IRTA-CReSA)
Freddy Dehesa-Canseco at ESPHM 2025
What is the poster about?
The poster presents the findings from a study investigating the presence of two types of bacteria – H. pylori and H. suis - in healthy pigs in Spain.
H. suis is a very common stomach bacterium in pigs, while H. pylori is primarily known for infecting humans, but has occasionally been detected in pigs.
The team from Vax2Muc partner IRTA-CReSA aimed to determine the frequency at which the two pathogens are detected in healthy pigs, to better understand the spread of these bacteria within pig populations. They collected samples across various Spanish commercial farms.
What are the main findings?
Researchers found that H. suis is frequently detected in pigs from swine farms in Spain. This microorganism was present in animals across all age groups, with a growing trend as the pigs get older. Surprisingly, in some cases, a 100 % detection rate was observed even in young pigs, while high percentages were also recorded in the other age groups. In contrast, H. pylori, a bacterium commonly found in humans, was not detected in any of the samples analysed.
How do these findings fit into the ongoing Vax2Muc research?
The evaluation of H. suis and H. pylori detection frequency in pigs from different farms and age groups is a key step in establishing a reliable preclinical porcine model for H. pylori. This stage is particularly important, as the animals selected for both the H. pylori infection model and the preclinical evaluation of the vaccine candidate should be ideally free from both pathogens.
Given the lack of scientific literature on the prevalence of these bacteria in the region where the experimental pigs were to be sourced, the researchers decided to analyse animals from multiple farms. This approach allowed them to determine the presence and frequency of H. suis and H. pylori, ensuring the selection of negative animals for the development of the Vax2Muc infection model.
Interested in finding out more?
You can download the poster here.
Conference: European Symposium of Porcine Health Management (ESPHM 2025), Bern, Switzerland
Poster title: Development of a preclinical Helicobacter pylori infection model in pigs using strains SS1 and J99
Presenter: Freddy Dehesa-Canseco, PhD student, Institute of Agrifood Research and Technology’s Animal Health Research Center (IRTA-CReSA)
Freddy Dehesa-Canseco at the ESPHM 2025
What is the poster about?
The poster presents the findings of a study that aimed to develop a preclinical model of H. pylori infection in pigs.
For the proper design, development, and evaluation of vaccines or medications, whether for humans or animals, it is essential to rely on preclinical models that allow the assessment of a product’s efficacy and functionality. These models represent a critical stage for observing biological responses before moving on to clinical trials.
In the case of mucosal vaccine research, pigs are considered a valuable preclinical (pre-human trial) model because their digestive system is physiologically and anatomically similar to that of humans.
The study used two strains of H. pylori (SS1 and J99), which were administered through the intragastric route to 12-week-old pigs. The objective was to establish an infection model in pigs that could later be used to evaluate the efficacy of a vaccine candidate against H. pylori.
Bacterial colonisation was assessed by collecting samples at various time points to determine whether the bacteria could be successfully detected in the animals, thereby confirming the establishment of the infection.
What are the main findings?
The study successfully reproduced a subclinical H. pylori infection model in pigs.
A subclinical infection model means that the infection was present in pigs, but it did not cause obvious sickness.
Researchers found that the J99 H. pylori strain was better at establishing a persistent infection in the pig’s stomachs compared to the SS1 strain and concluded that the J99 strain is likely the most suitable for future studies evaluating vaccine effectiveness and other treatments.
How do these findings fit into the ongoing Vax2Muc research?
These findings are relevant to the Vax2Muc project because they support the establishment of a subclinical infection model in pigs, which is essential for studying the behaviour of H. pylori in a living organism. This model will be used in the future to evaluate the efficacy of the vaccine candidate under controlled conditions, providing a solid foundation for its preclinical development.
Interested in finding out more?
You can download the poster here.