Vax2Muc research highlights: From vaccine biodistribution to AI tools
The Vax2Muc consortium aims to develop next-generation vaccines against diseases caused by mucosal pathogens colonising the gastrointestinal tract. More concretely, the goal of this interdisciplinary research collaboration is to create, as a proof-of-concept, a prophylactic H. pylori vaccine candidate that will be evaluated in a phase I clinical trial.
Exciting findings are emerging from the ongoing research by the international, multidisciplinary consortium, and researchers are busy presenting them at conferences. In the second edition of Vax2Muc research highlights, we feature the four posters presented in the second half of 2025 by two of the project’s PhD students – Yueqi Wang from the University of Strathclyde and Martina Bardino Mørch from Statens Serum Institut. Their work reflects the consortium’s commitment to exploring the best ways a vaccine could be delivered to the stomach and be effective in this overall inhospitable environment.
Conference: CRS 2025 Annual meeting, Philadelphia, USA
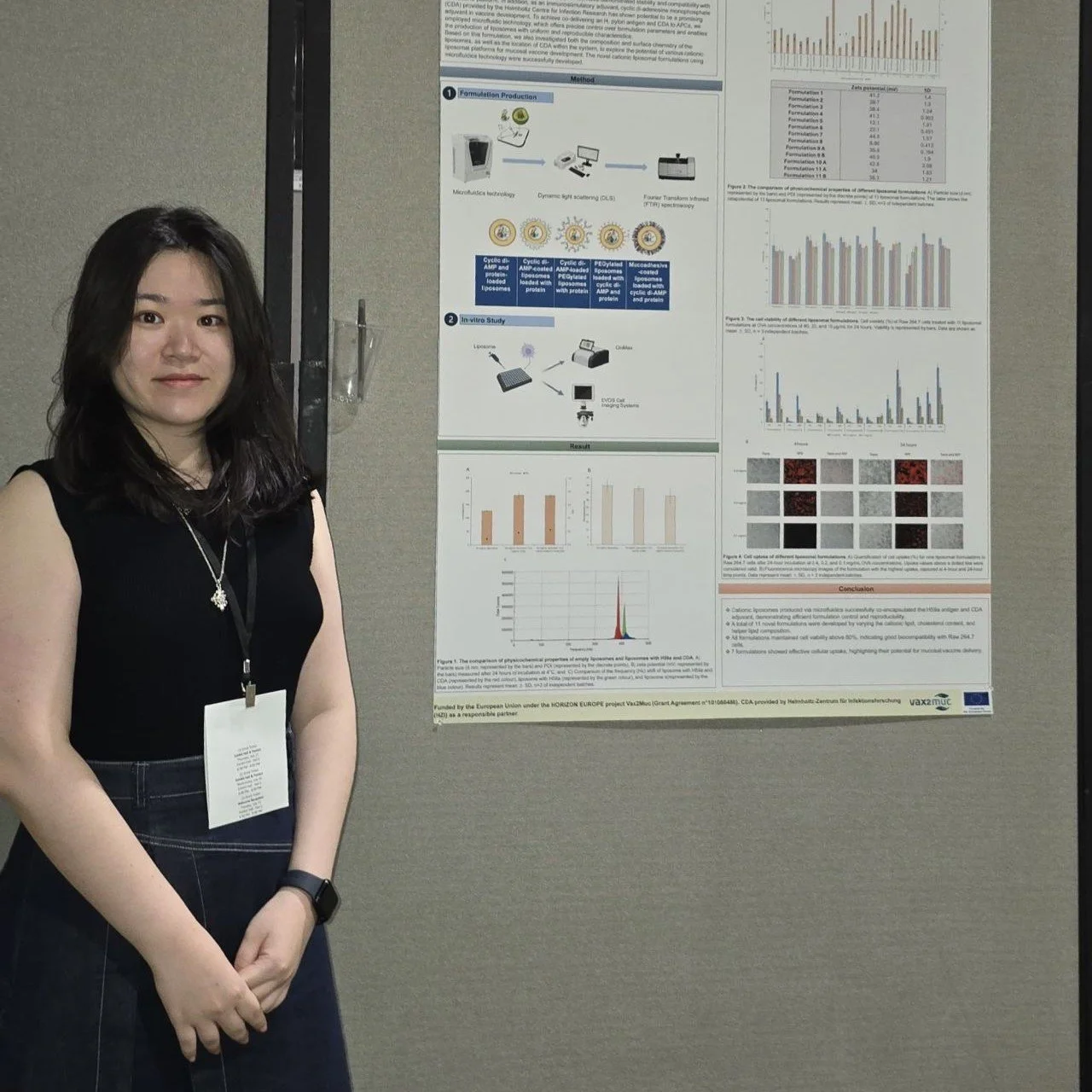
Poster title: Microfluidic manufacturing of liposomal adjuvants
Presenter: Yueqi Wang, PhD student, University of Strathclyde
What is this poster about?
The poster summarises the findings from recent research aimed at developing effective delivery methods for a mucosal vaccine against pathogens such as H. pylori. Specifically, the researchers developed cationic liposomes to deliver the vaccine directly to the immune cells in the gastrointestinal tract. Cationic liposomes are tiny, fatty bubbles which can act as delivery packages for vaccines.
Researchers developed 11 different versions of these packages for testing. They used microfluidic technology, a method that enables precise ingredient mixing, ensuring uniform production every time.
What are the main findings?
Results show that the tested cationic liposome formulations were biocompatible, meaning that they did not kill the cells they were tested on. In 7 of the 11 formulations, they found that the cells successfully took up the vaccine.
The research concludes that microfluidic technology is an effective approach for producing stable cationic liposomes for mucosal vaccine delivery.
How do these findings fit into the ongoing Vax2Muc research?
The Vax2Muc consortium aims to develop a mucosal vaccine against H. pylori that would trigger local immunity in the stomach, where such pathogens enter the body. Exploring the best ways to deliver vaccines to the stomach – including by using cationic liposomes - is essential for this process.
Interested to learn more?
Download the poster here.
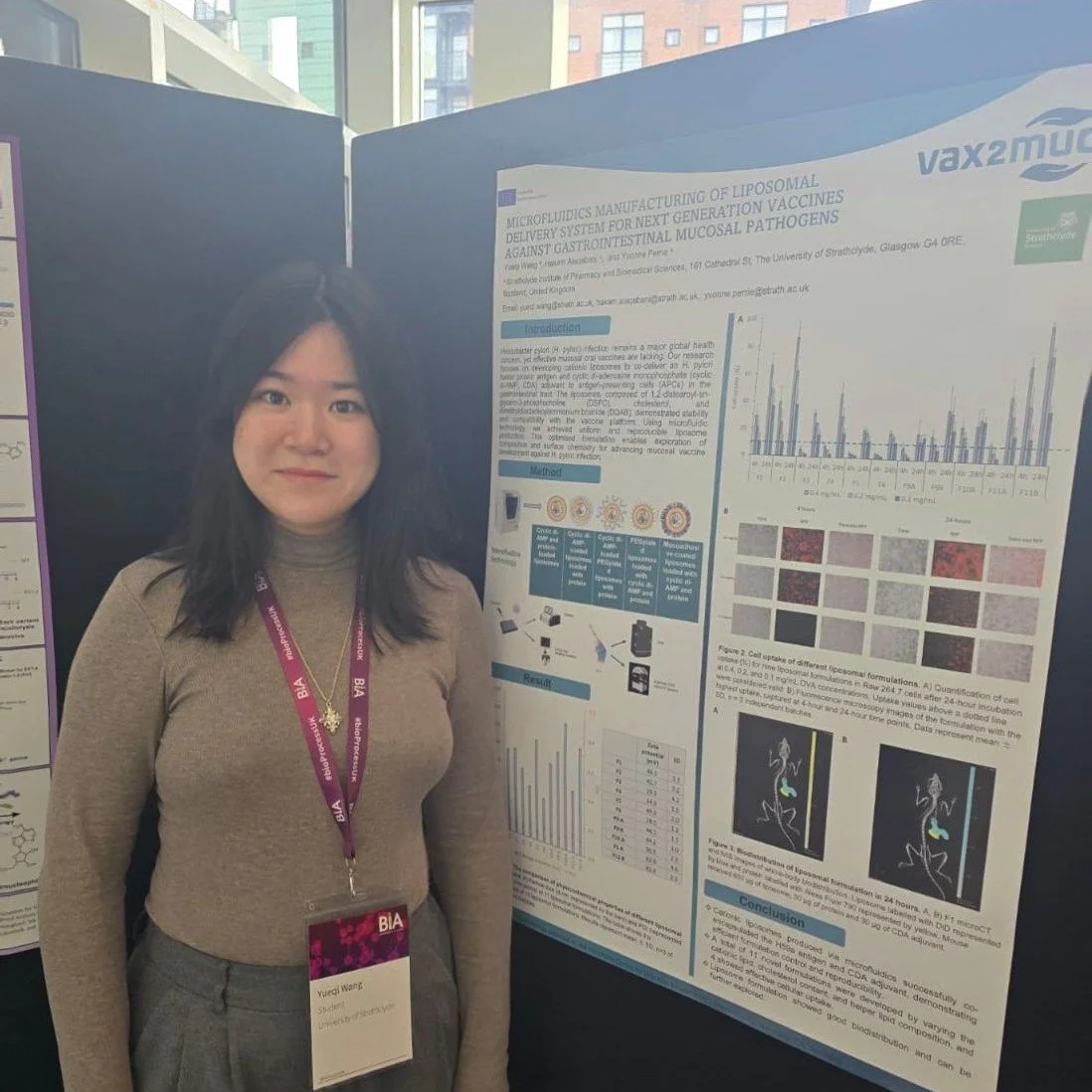
A few months later, Yueqi Wang also presented this work at the BioProcessUK Conference 2025 in Brighton, UK, with the addition of findings from a then-finished mouse study.
What are the new findings?
The mouse study reveals the biodistribution of vaccines to explore exactly where the vaccine went and how long it remained in the body after being swallowed.
Researchers used fluorescent labels to differentiate between the vaccine antigen and the delivery vehicle (cationic liposomes). They monitored the mice over 24 hours and found that the liposome formulation showed good biodistribution, indicating that the vaccine reached the intended areas of the gastrointestinal tract.
Interested to learn more?
Download the poster here.
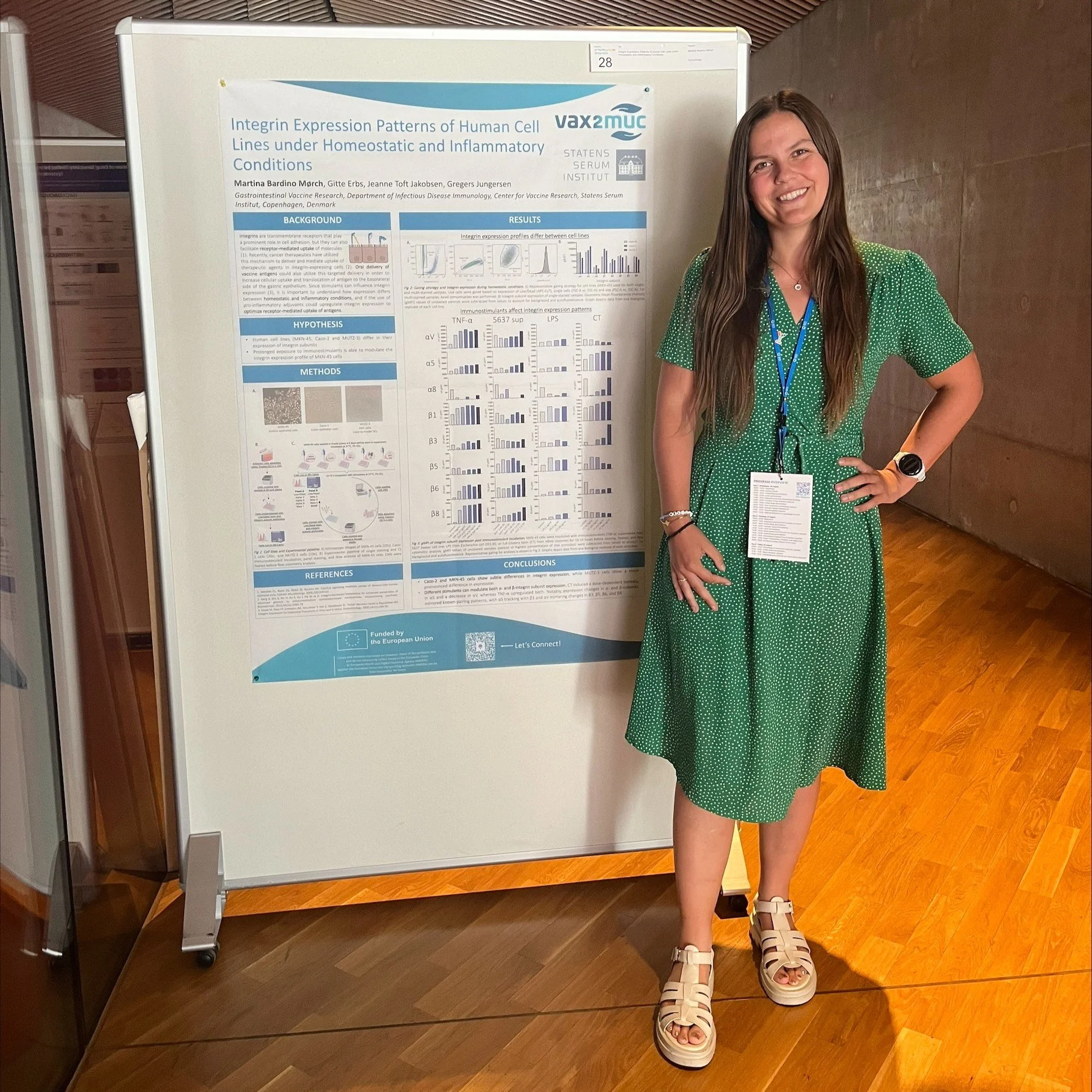
Conferences: Nordic Flow Cytometry Meeting 2025, Copenhagen, Denmark, and the 19th Vaccine Congress, Kyoto, Japan
Poster title: Integrin Expression Patterns of Human Cell Lines under Homeostatic and Inflammatory Conditions
Presenter: Martina Bardino Mørch, PhD student, Statens Serum Institut
What is the poster about?
The poster presents the findings from research on integrins and how they change depending on whether the body is in a healthy or an inflamed state.
Integrins are proteins on the cell surface that usually help cells stick together, but can also act as receptors that swallow up specific molecules.
Researchers explored whether integrins could be used to target vaccines to the right cells. They used three types of human cells to determine how many integrin receptors were present. Then they exposed these cells to various triggers, such as toxins or inflammatory signals, to see whether they would induce the cells to produce more receptors.
What are the main findings?
Researchers found that inflammation and different stimulants can change the profile of these receptors. The authors conclude that understanding how these changes emerge during inflammation could help scientists optimise how vaccines are absorbed by the body, potentially making them more effective.
How do these findings fit into the ongoing Vax2Muc research?
Understanding how mucosal epithelial cells respond to different triggers contributes to efforts to optimise vaccine design and vaccine absorption in the human stomach, thereby making future oral vaccines more effective.
Interested to learn more?
Download the poster here.
Conference: Annual meeting in immunology and infectious diseases, Copenhagen, Denmark
Poster title: De Novo Protein Design with RFdiffusion: A New Paradigm for Rational Vaccine Antigen and Adjuvant Development
Presenter: Martina Bardino Mørch, PhD student, Statens Serum Institut
Martina Bardino Mørch at University of Copenhagen’s annual meeting in immunology and infectious diseases
What is the poster about?
This poster presents new, AI-supported methods researchers are using to engineer vaccine components.
So far, only a small number of adjuvants have been approved for human use, underscoring the need for new, innovative methods. Using AI, the discovery of new molecules for mucosal adjuvants can be turned into a design process rather than the tedious trial-and-error of traditional research.
Adjuvants are ingredients in a vaccine that enhance the immune response to the antigen.
The researchers behind this work propose a new approach to vaccine development that uses various AI tools to build vaccine components.
They used three different AI tools and presented their use in protein design:
· RFdiffusion creates the basic backbone of a new protein.
· ProteinMPNN decides which specific building blocks (amino acids) are needed to make the shape stable.
· AlphaFold predicts how the new protein will actually fold in 3D space.
What are the main findings?
The authors believe these computer designs are an exciting starting point for a new approach in vaccine research. One concrete way it can be used is to design more effective adjuvants.
They also emphasise the need for continuous rigorous biological testing to translate these new designs into reliable immunological tools.
How do these findings fit into the ongoing Vax2Muc research?
The Vax2Muc consortium’s ongoing research is focused on developing a mucosal vaccine against H. pylori.
These AI-supported methods present a potential shift from trial-and-error methods in vaccine development towards rational, structured design, allowing for the deliberate engineering of vaccine ingredients to be more effective, stable, and targeted. These new tools could speed up research and open new, previously difficult-to-explore approaches in vaccine design.
Interested to learn more?
Download the poster here.